Novel quinoline-derived mTOR inhibitors with remarkable enzymatic and cellular activities: design, synthesis and biological evaluation
Abstract
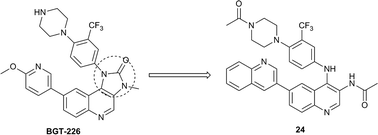
Herein, we reported the preparation and in vitro development of a novel series of quinoline-based mTOR inhibitors, some of which were obtained via introducing a ring-opening strategy. As for enzymatic activity, more than half of these quinoline derivatives exhibited moderate to potent inhibition against mTOR. Among them, six compounds showed IC50 values below 50 nM. In particular, several quinolines exhibited remarkably enhanced anti-proliferative activities against all the three tested tumor cell lines in contrast to the initial lead 9. As a representative in this series, compound 24 demonstrated IC50 values of 0.11, 0.17 and 0.04 μM against HCT-116, PC-3 and MCF-7 cell lines, respectively. Besides, compounds 17 and 24 were identified to be selective over class I PI3Ks. Further Western blot analysis validated the dual inhibition of mTORC1 and mTORC2 as a result of compound 24 treatment in the MCF-7 cell line, which was beneficial for conquering the S6K/IRS1/PI3K negative feedback loop. Moreover, acceptable stability was displayed by compound 17, another representative of this series, in simulated intestinal fluid (SIF), simulated gastric fluid (SGF), as well as rat liver microsome (RLM). By virtue of the favorable biological profiles, several quinolines merit further in vivo investigation.

 Please wait while we load your content...
Please wait while we load your content...