Syntheses and biological evaluations of highly functionalized hydroxamate containing and N-methylthio monobactams as anti-tuberculosis and β-lactamase inhibitory agents†‡
Abstract
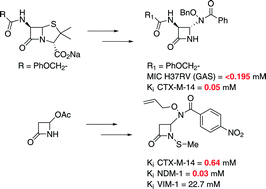
Both the resurgence of tuberculosis (TB) and antibiotic resistance continue to threaten modern healthcare and new means of combating pathogenic bacterial infections are needed. The syntheses of monobactams possessing hydroxamate and N-methylthio functionality are described, as well as their anti-TB, in vitro β-lactamase inhibitory, and general antimicrobial evaluations. A number of compounds exhibited significant anti-TB and β-lactamase inhibitory activity, with MIC values in the range of 25 to <0.19 μM against Mycobacteria tuberculosis (M. tb), and Ki values in the range of 25–0.03 μM against purified NDM-1 and VIM-1 lystate metallo β-lactamases. This work suggests that these scaffolds may serve as promising leads in developing new antibiotics and/or β-lactamase inhibitors.

 Please wait while we load your content...
Please wait while we load your content...