Inhibition of phosphatidylinositol-3,4,5-trisphosphate binding to the AKT pleckstrin homology domain by 4-amino-1,2,5-oxadiazole derivatives†
Abstract
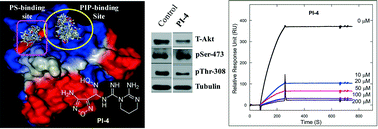
Aberrant regulation of phosphatidylinositol-3-kinase (PI3K)-dependent cell signalling pathways is directly linked with different human cancers. AKT is a phosphatidylinositol (PIP) binding serine/threonine kinase enzyme and a key component of PI3K signalling pathways. Several efforts have been made to perturb the activities of AKT enzymes; however, the selectivity issue remains a key challenging point. The pleckstrin homology (PH) domain of AKT strongly interacts with the membrane-localized PIPs and activates the enzyme. Several attempts are underway for the development of small molecules, which can specifically inhibit the PIP/PH domain interaction to ascertain an alternative approach for the development of drug(s) for PI3K–AKT signalling pathways. This proposed approach is highly beneficial because of easy synthesis of small molecules and low side effects. In this study, we used a series of small molecule antagonists (PIs) for PI(3,4,5)P3/PH domain interaction and determined their inhibitory effect by performing competitive surface plasmon resonance (SPR) analysis (IC50 ranges from 1.85 to 16.35 μM for the PI(3,4,5)P3/AKT1-PH domain binding assay). To elucidate their binding selectivity, we also used PI(3,4,5)P3, PI(3,4)P2, and PI(4,5)P2 specific PH domains. To further understand their PH domain inhibition mechanism, we also performed various physicochemical analyses. The results showed that these water-soluble compounds do not significantly interact with the model membranes. The oxadiazole and N′-hydroxy moieties of the compounds are essential for their exothermic interaction with the PH domains and their binding does not alter the secondary structure of the PH domain. Potent compounds show a moderate effect on the phosphothreonine (308) level of the AKT enzyme. Overall, our studies demonstrate that these compounds could interact with the PIP-binding PH domains and inhibit their membrane recruitment. These studies also illustrate the feasibility of further development of 4-amino-N′-hydroxy-1,2,5-oxadiazole-3-carboximidamide moiety-based small molecule antagonists for PI3K signalling pathways.

 Please wait while we load your content...
Please wait while we load your content...