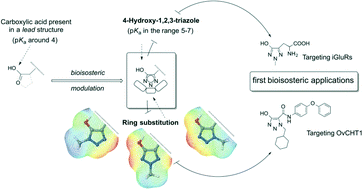
Bioisosterism and scaffold hopping are two widely used approaches in medicinal chemistry for the purpose of lead optimization. The study highlights the physicochemical properties of the 4-hydroxy-1,2,3-triazole scaffold, a less investigated heterocyclic system. Synthetic strategies to obtain different N-substituted 4-hydroxy-1,2,3-triazole isomers are presented, and their role as possible isosteres of the carboxylic acid is discussed. The aim is to use this system to modulate the acidic moieties present in lead compounds and, at the same time, to regiodirect substituents in set directions, through targeted substitution on the three nitrogenatoms of the triazole ring. Through this approach, compounds having enhanced binding affinity, will be sought. Two examples of bioisosteric applications of this moiety are presented. In the first example, a classical bioisosteric approach mimicking the distal (S)-glutamic acid carboxyl group using the 4-hydroxy-1,2,3-triazole moiety is applied, to obtain two promising glutamate analogs. In the second example, a scaffold hopping approach is applied, replacing the phenolic moiety present in MDG-1-33A, a potent inhibitor of Onchocerca volvulus chitinase, with the 4-hydroxy-1,2,3-triazole scaffold. The 4-hydroxy-1,2,3-triazole system appears to be useful and versatile in drug design.


 Please wait while we load your content...
Please wait while we load your content...