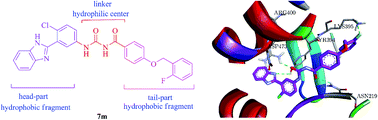
A series of novel N-3-benzimidazolephenylbisamide derivatives bearing the 4-benzyloxyphenyl moiety were synthesized and evaluated for their antiproliferative activity against MGC803, HT29, MKN45 and SW620 cancer cell lines in vitro. The pharmacological data demonstrated that the majority of the target compounds exhibited higher efficacy against MGC803, HT29 and MKN45 cells, among which compound 7m displayed higher antiproliferative activity than vismodegib. Furthermore, compound 7m manifested better inhibition of the Hedgehog (Hh) signaling pathway in different Hh-related assays and may compete with boron-dipyrromethene (BODIPY)-cyclopamine for binding to the human smoothened (Smo) receptor. In addition, molecular docking studies suggested that compound 7m interacts very closely with the vismodegib docking pose through hydrogen bonds at the taladegib binding site of the Smo receptor.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...