3-Propionyl-thiazolidine-4-carboxylic acid ethyl esters: a family of antiproliferative thiazolidines†
Abstract
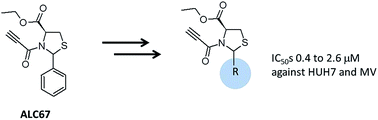
Cancer results from unregulated cell growth. Reactivating the process of the programmed cell death, i.e. apoptosis, is a classical anticancer therapeutic strategy. The apoptosis-inducing property of the (2RS,4R)-2-phenyl-3-propionyl-thiazolidine-4-carboxylic acid ethyl ester (

 Please wait while we load your content...
Please wait while we load your content...