Nature of the charged head group dictates the anticancer potential of lithocholic acid-tamoxifen conjugates for breast cancer therapy†
Abstract
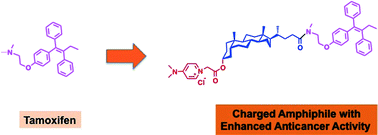
Modulation of existing drugs is required to achieve enhanced activity for cancer therapy by lowering their effective dose. Strategies for introduction of cationic charge and hydrophobicity have been proposed and explored to enhance the therapeutic effects of anticancer drugs. In this manuscript, we planned modulation of tamoxifen and synthesized eight tamoxifen (Tam) conjugated lithocholic acid (LCA) amphiphiles with variable cationic charged head groups. We determined the anticancer potential of these amphiphiles against different breast cancer cell lines. Activity of these amphiphiles is contingent on nature of the charged head group, as hard-charged amphiphiles exhibit strong membrane interactions and enhanced anticancer activity compared to soft-charged amphiphiles. In-depth mechanistic studies concluded that conjugation of a dimethyl amino pyridine (DMAP) charged head group in case of LCA-Tam-DMAP enhances therapeutic effect of Tam in breast cancer cells, and makes it highly effective even against ER negative cells. Amphiphilic character of these lipid-drug conjugates can be further explored for engineering nanotherapeutics for targeting tumors. Therefore, fine-tuning the interactions of drugs with cell membranes can help in engineering future lipid-drug conjugates for effective cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...