Macrocyclic derivatives of 6-methyluracil as ligands of the peripheral anionic site of acetylcholinesterase†
Abstract
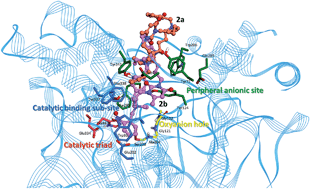
Novel pyrimidinophanes possessing two o-nitrobenzylethyldialkylammonium heads bridging with different spacers were prepared. Pyrimidinophanes 2a, 2b and 3 are reversible inhibitors of cholinesterases. They show a very good selectivity for human acetylcholinesterase (AChE), with an inhibitory power 100–200 times higher than for human butyrylcholinesterase (BChE). Docking simulations indicate specific binding of pyrimidinophanes 2a and 4 onto the peripheral anionic site of AChE. Other compounds bind to the active center of AChE as well as to the peripheral anionic site. These compounds are dual binding site inhibitors. Pyrimidinophane 2b and its acyclic counterpart 1 were tested in the animal model of myasthenia gravis and may be considered as valuable candidates for the treatment of pathological muscle weakness syndromes.

 Please wait while we load your content...
Please wait while we load your content...