Theoretical and experimental investigation on clarithromycin, erythromycin A and azithromycin and descladinosyl derivatives of clarithromycin and azithromycin with 3-O substitution as anti-bacterial agents†
Abstract
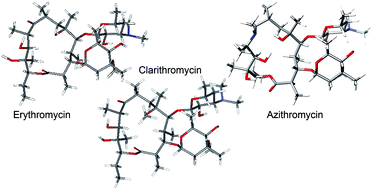
Erythromycin A, clarithromycin and azithromycin are the three most important macrolide antibiotics and are all very widely used in the clinic. They act on the bacterial ribosome inhibiting protein synthesis. We have performed both NMR (transferred NOESY) and modelling studies in order to determine the conformations of these antibiotics when they are bound to ribosomes. All three drugs exhibit the “folded-out” conformation in the bound state, but the dominant conformation of azithromycin is not completely superimposable on the clarithromycin and erythromycin A 9-ketone structures. Modelling suggests that clarithromycin (based on a 14-membered ring) and its 3-O-substituted descladinosyl derivatives are conformationally rigid molecules; these are compounds which generally exhibit more activity against Gram-positive bacteria. Azithromycin (based on a 15-membered ring) and its 3-O-descladinosyl derivatives are flexible in silico and show more activity against Gram-negative bacteria in culture.

 Please wait while we load your content...
Please wait while we load your content...