Synthesis of cyclohexapeptides as antimalarial and anti-trypanosomal agents†
Abstract
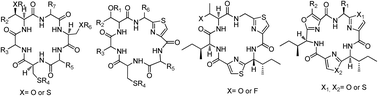
Cyclohexapeptide analogs of natural products were obtained in very good yields by a combination of solid-phase peptide synthesis, for the linear peptide, and solution cyclization. The activities against Plasmodium falciparum K1, Trypanosoma brucei brucei and murine macrophages (cell line J774) of these novel compounds and azolic macrocycles, previously reported by us, were evaluated. Seven macrocycles showed submicromolar activities against Plasmodium falciparum K1 and a high selectivity (SI > 125) for the parasite. In addition, two compounds displayed one digit micromolar EC50 against T. brucei brucei and satisfactory selectivity (SI 82 and 95). Preliminary structure–activity relationships are presented.

 Please wait while we load your content...
Please wait while we load your content...