Neoglycosylation and neoglycorandomization: enabling tools for the discovery of novel glycosylated bioactive probes and early stage leads
Abstract
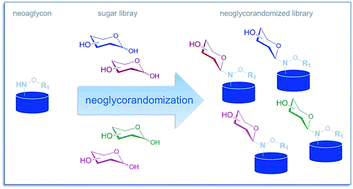
This review focuses upon the development, scope, and utility of the highly versatile chemoselective alkoxyamine-based ‘neoglycosylation’ reaction first described by Peri and Dumy. The fundamentals of neoglycosylation and the subsequent development of a ‘neoglycorandomization’ platform to afford differentially-glycosylated libraries of plant-based natural products, microbial-based natural products, and small molecule-based drugs for drug discovery applications are discussed.

 Please wait while we load your content...
Please wait while we load your content...