Design of glycosyltransferase inhibitors targeting human O-GlcNAc transferase (OGT)†
Abstract
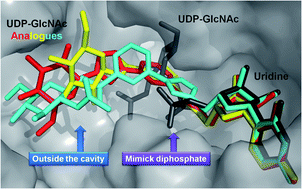
Inhibition of glycosyltransferases requires the design of neutral inhibitors to allow cell permeation in contrast to their natural dianionic substrates. O-GlcNAc transferase (OGT) is a key enzyme involved in dynamic glycosylation of cytosolic and nuclear proteins in competition with phosphorylation. Designing OGT inhibitors is of prime interest for the better understanding of its biological implications. Introduction of a pyridine moiety as a pyrophosphate surrogate was evaluated, which provided moderate in vitro inhibition of OGT. Docking studies highlighted some key features for the binding of the designed inhibitors to the catalytic site of OGT where the carbohydrate moiety did not occupy its natural position but rather turned away and pointed to the solvent outside the catalytic pocket. Further investigation with cellular assays did not provide inhibition of OGT. This lack of OGT inhibition was rationalized with a permeation assay which revealed the sequestration of the inhibitors at the membrane.

 Please wait while we load your content...
Please wait while we load your content...