The chemistry and biology of the α-ketoglutarate-dependent histone Nε-methyl-lysine demethylases
Abstract
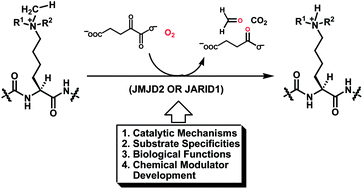
Since the identification of the Jumonji C-domain-containing α-ketoglutarate-dependent histone Nε-methyl-lysine demethylases in 2006, exemplified with the JMJD2 (Jumonji domain-containing protein 2) and JARID1 (Jumonji, AT rich interactive domain 1) families of the Nε-trimethyl-lysine demethylases, there has been a steady increase in our functional understanding of these two families of the non-heme hydroxylase enzymes which achieve Nε-methyl-lysine demethylation via the prior Nε-CH3 C-hydroxylation. These enzymatic demethylation reactions have been shown to constitute critical epigenetic regulatory mechanisms and have been proposed as novel cancer therapeutic targets. Therefore, we have also seen medicinal chemistry efforts during the past few years toward identifying potent and selective inhibitors for the demethylation reactions catalyzed by the JMJD2 and JARID1 proteins. In the meantime, biochemical and structure-based mechanistic investigations have also furnished molecular insights of the catalysis and substrate recognition for these demethylation reactions. An account on the current knowledge of the chemistry and biology of the demethylation reactions catalyzed by the JMJD2 and JARID1 families of demethylases will be presented in this review. It is hoped that this article will also be able to set a stage for future research at the interface of chemistry and biology on the demethylation reactions catalyzed by these fascinating enzymes.

 Please wait while we load your content...
Please wait while we load your content...