Differential activation of soluble guanylate cyclase by a series of aryl disulfanyl dinitrate esters
Abstract
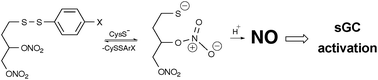
Classic organic nitrates, such as nitroglycerin (GTN), are important vasodilators classed as NO donors, since biological activity is assumed to result entirely from bioactivation to NO and activation of soluble guanylate cyclase (sGC). Para-substituted aryl disulfanyl nitrate esters (SS-nitrates), designed based on a sulfhydryl-dependent mechanism of NO release, were investigated for their sGC activation. To obtain further insights into their interaction with hemoproteins, SS-nitrates were reacted with oxyhemoglobin (O2-Hb). SS-nitrates activated sGC with higher efficacy than GTN in the presence of cysteine. Some SS-nitrates, unlike GTN, activated sGC in the absence of cysteine. Linear correlations between the σp Hammett parameters and sGC activation efficacies or rates of O2-Hb oxidation by SS-nitrates in the presence of cysteine were found. Our results validate the design of SS-nitrates to circumvent the need for cysteine-mediated bioactivation and hint at NO-dependent and NO-independent mechanisms of sGC activation.

 Please wait while we load your content...
Please wait while we load your content...