2-Hydroxy-substituted cinnamic acids and acetanilides are selective growth inhibitors of Mycobacterium tuberculosis†
Abstract
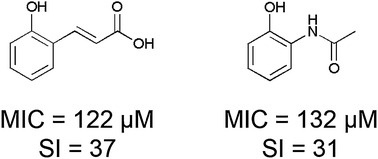
Selective chemical hits are required for feeding the initial discovery phase of the anti-tuberculosis therapeutics pipeline. These chemical entities should ideally target novel mechanisms of action in order to tackle drug resistance in Mycobacterium tuberculosis. In this work, hydroxycinnamic acid and acetamidophenol skeleta were employed for assessing the effects of constitutional isomerism on in vitro anti-TB activity. The whole cell evaluation of minimum inhibitory concentration values of different substituted cinnamic acids and acetamidophenols showed that the free ortho hydroxyl group conferred both potency and selectivity. Both 2-coumaric acid and 2-acetamidophenol showed minimum inhibitory concentration below 150 μM against M. tuberculosis H37Rv and selectivity index higher than 30.

 Please wait while we load your content...
Please wait while we load your content...