Optimising pharmacokinetics of glucokinase activators with matched triplicate design sets – the discovery of AZD3651 and AZD9485†
Abstract
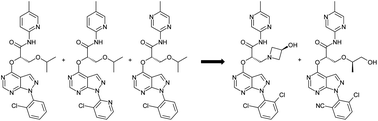
The matched triplicate approach to lead optimisation offers a means of generating more robust quantitative structure activity relationship data and this rigour leads to better quality decision making and greater ability to predict optimal compounds within a series. One of the ultimate aims of this approach is to use the data generated to build more accurate predictive models to identify the best compounds within the exemplified chemical space in an efficient manner. This paper describes the continued application of this approach to the optimisation of a series of glucokinase activators. This second phase focussed primarily on the rational solution to plasma instability observed with the previous compounds and, hence, achieved acceptable oral exposure in the series. The campaign was completed by using the predictive power of Free-Wilson analysis based on the matched triplicate datasets to enable a focussed, matrix based endgame culminating in the identification of two development candidates, AZD3651 and AZD9485.

 Please wait while we load your content...
Please wait while we load your content...