Structure-activity evaluation of new uracil-based non-nucleoside inhibitors of HIV reverse transcriptase†
Abstract
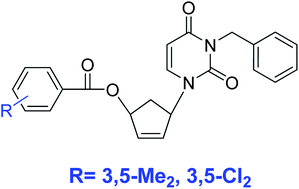
A new series of potential nonnucleoside inhibitors of HIV-1 reverse transcriptase (NNRTIs) based on the carbocyclic 5′-nor-uracil scaffold were designed and synthesized. Three different aspects of the scaffold were investigated: the effects of adding a linker between the carbocyclic and phenyl fragments, introduction of different substituents on the benzoyl residue and replacing the central carbocyclic ring with a benzyl group. Analogues of 2′,3′-dideoxy-2′,3′-didehydro-5′-nor-uridine, bearing 3,5-dichloro- or 3,5-dimethylbenzoyl groups, showed inhibitory activity against HIV-RT wild-type (IC50 5–10 μM) and mutants L100I (IC50 1.2–2.1 μM) and K103N (IC50 8–17 μM).
 Please wait while we load your content...
Please wait while we load your content...