Synthesis and biological evaluation of novel 2,3-dihydro-1H-1,5-benzodiazepin-2-ones; potential imaging agents of the metabotropic glutamate 2 receptor†
Abstract
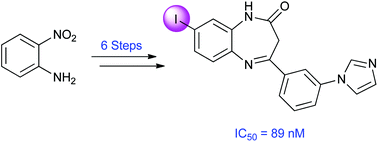
A focused library of novel 2,3-dihydro-1H-1,5-benzodiazepin-2-ones containing sites for 11C-, 18F- and 123I-labelling have been prepared and evaluated against membrane expressing human recombinant metabotropic glutamate 2 receptor (mGluR2). The compounds were found to be non-competitive

 Please wait while we load your content...
Please wait while we load your content...