Synthesis and in vitro pharmacological evaluation of indolyl carboxylic amide analogues as D3 dopamine receptor selective ligands†
Abstract
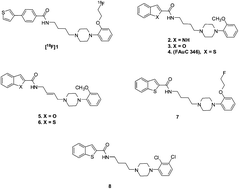
A series of substituted 1H-indolyl carboxylic acid amides that contain a N-(2-methoxyphenyl)piperazine or N-(2-fluoroethoxy)piperazine group were synthesized and their affinities for human dopamine D2, D3, and D4 receptors were determined. Two of these compounds, 14a and 14b, displayed high binding affinity at D3 (Ki = 0.18 and 0.4 nM, respectively), and selectivity for D3vs. D2 receptors (87-fold and 60-fold, respectively). These two compounds had low binding affinity at D4 receptors and σ receptor sites. The intrinsic activity of these compounds at D2 and D3 receptors was determined using a forskolin-dependent adenylyl cyclase inhibition assay; both 14a and 14b were found to be partial agonists. Furthermore, for compound 14a, the log D value of 2.85 suggested it has suitable lipophilicity for crossing the blood–brain-barrier.

 Please wait while we load your content...
Please wait while we load your content...