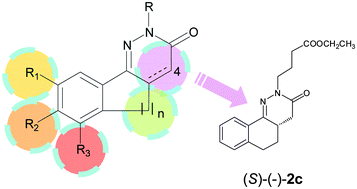
Through a cell-based biological screening, the benzocinnolinone derivative (±)-2c was identified as a promising STAT3 inhibitor. Since SAR studies on a series of compounds structurally related to (±)-2c (1c, 2a–p, 3c, 4c, 6) showed that the latter had the most significant inhibitory activity, we investigated in depth its essential structural features. In particular, enantiomeric separation was performed, and the absolute configuration of the stereoisomers was assigned by theoretical and crystallographic studies. The biological evaluation highlighted that (S)-(−)-2c is twice as potent as (R)-(+)-2c.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Please wait while we load your content...
Please wait while we load your content...