Mannich- and Lederer–Manasse-based analogues of the natural product S-(+)-curcuphenol as cancer proliferation and migration inhibitors†
Abstract
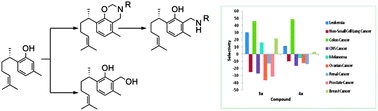
The Mannich-based analogues of S-(+)-curcuphenol (1), 3a–5a, and 3b potently inhibited the growth of several NCI's human cancer cell lines. In addition, 7a showed a 12-fold antimigratory activity (IC50 3.1 μM) against the human breast cancer cell line MDA-MB-231 compared to its parent 1 (IC50 37.2 μM).

 Please wait while we load your content...
Please wait while we load your content...