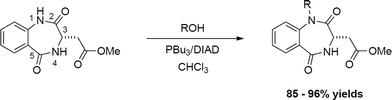
Fine-tuning the chemo- and regioselective alkylation of 1,4-benzodiazepines: further applications of the Mitsunobu reaction†
Abstract
The reaction of 1,4-benzodiazepines with primary and secondary alkyl bromides/iodides can lead to a mixture of N- and O-alkylations of both the

 Please wait while we load your content...
Please wait while we load your content...