Design and synthesis of novel N,N′-glycoside derivatives of 3,3′-diindolylmethanes as potential antiproliferative agents†
Abstract
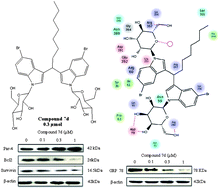
A library of 34 compounds containing the DIM core have been synthesized and tested for their anticancer efficacy by measuring their cytotoxicity to cancer cell lines A549, HeLa and MCF-7. Some of the selected derivatives were N-glycosylated to increase their efficacy. Compound 7d, an N-glycosylated DIM derivative, was found to be effective at 1.3, 0.3 and 0.9 μmol concentrations against A549, HeLa and MCF-7, respectively. Immunochemistry studies revealed that it could induce apoptosis by upregulating a pro-apoptotic protein Par-4 and concomitantly diminishing the expression of pro-survival proteins Bcl-2 and GRP78. Flow cytometry studies showed that the compound arrested cells in the G1 phase of the cell cycle and significantly abrogated the motility of HeLa cells. Computer docking simulations of 7d with GRP78 suggested its involvement in two H-bonds with Asp78, two H-bonds with Arg290, one with Arg367, and one water mediated H-bond interaction. The interaction patterns also demonstrated that the presence of bromide in the vicinity (within 3.5 Å) of Lys294 generates the possibility of a halogen bond, which may also contribute in providing some extra stability to the complex. Hence, compounds of this class will be useful for the design of new anticancer agents.

 Please wait while we load your content...
Please wait while we load your content...