‘Light up’ protein–protein interaction through bioorthogonal incorporation of a turn-on fluorescent probe into β-lactamase†
Abstract
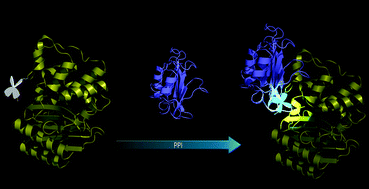
Fluorescent labeling of biomacromolecules to ‘light up’ biological events through non-invasive methods is of great importance, but is still challenging in terms of fluorophore properties and the labeling methods used. Herein, we designed and synthesized a biocompatible and conformation sensitive tetraphenylethene derivative EPB with aggregation induced emission (AIE) properties. By introducing EPB into TEM-1 β-lactamase (TEM-1 Bla) through a two-step approach, a conformation-dependent fluorescent sensor EPB104-Bla was genetically engineered, which was applied to monitor the protein–protein interaction (PPI) with β-lactamase inhibitor protein (BLIP). The fluorescence signal of EPB104-Bla increases by an approximately 5-fold upon binding to BLIP, indicating that EPB-104 Bla is capable of lighting up the PPI. The dissociation constant (Kd) between EPB104-Bla and BLIP was estimated to be 0.6 μM, which is consistent with that derived from the kinetic inhibition assay. This study demonstrates that genetic modification of proteins with AIE probes might open up new opportunities to develop biosensors in PPI analysis.


 Please wait while we load your content...
Please wait while we load your content...