S95C substitution in CuZn-SOD of Ipomoea carnea: impact on the structure, function and stability†
Abstract
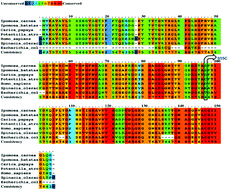
Superoxide dismutase (SOD) in general is a unique homo-dimeric enzyme that can scavenge toxic superoxide radicals by dismutation reaction. In IcSOD (Ipomoea carnea SOD), the presence of cysteine (Cys) plays an essential role in protein behaviour. This study analysed the role of Cys in modulating the stability and kinetic properties of IcSOD. To investigate the significance of the dimeric structure in modulating the structure/function relationship of CuZn-SODs, we have substituted a conserved serine by cysteine (Ser95Cys) in Ipomoea carnea CuZn-SOD. The results demonstrate that this mutation leads to an increase in dimeric strength, as reflected by size exclusion chromatography, differential scanning calorimetry, and high-temperature circular dichroism spectroscopy measurements. The mutant form, as compared to the native enzyme, shows a relatively low tendency to form aggregates but encountered a reduction in both dismutase and peroxidase activities. This study provides new mechanistic insight into the role of free cysteine in CuZn-SODs and such mutation may be used to increase dimeric strength. Protein docking and molecular dynamics simulations further demonstrate that Ser95Cys substitution in Ipomoea carnea CuZn-SOD leads to the creation of a new subunit interface resulting in increased dimeric strength of the protein.

 Please wait while we load your content...
Please wait while we load your content...