Exploring the origin of the catalytic power and product specificity of SET domain protein methyltransferase†
Abstract
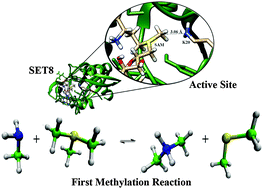
Herein, we used computer simulation to evaluate the free energy activation barriers of the first and second methyl transfer for native SET8 PKMT and its Y334F mutant. The results suggest that the origin of SET8 catalytic power is mainly due to electrostatic preorganization.

 Please wait while we load your content...
Please wait while we load your content...