Differential quantification of isobaric phosphopeptides using data-independent acquisition mass spectrometry†
Abstract
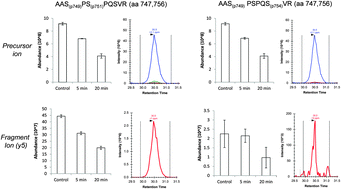
Phosphorylation is a post-translational modification (PTM) fundamental for processes such as signal transduction and enzyme activity. We propose to apply data-independent acquisition (DIA) using mass spectrometry (MS) to determine unexplored phosphorylation events on isobarically modified peptides. Such peptides are commonly not quantitatively discriminated in phosphoproteomics due to their identical mass.

 Please wait while we load your content...
Please wait while we load your content...