The unique functional role of the C–H⋯S hydrogen bond in the substrate specificity and enzyme catalysis of type 1 methionine aminopeptidase†
Abstract
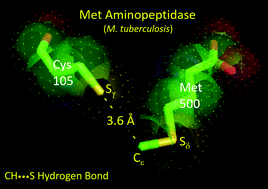
It is intriguing how nature attains recognition specificity between molecular interfaces where there is no apparent scope for classical hydrogen bonding or polar interactions. Methionine aminopeptidase (MetAP) is one such enzyme where this fascinating conundrum is at play. In this study, we demonstrate that a unique C–H⋯S hydrogen bond exists between the enzyme methionine aminopeptidase (MetAP) and its N-terminal-methionine polypeptide substrate, which allows specific interaction between apparent apolar interfaces, imposing a strict substrate recognition specificity and efficient catalysis, a feature replicated in Type I MetAPs across all kingdoms of life. We evidence this evolutionarily conserved C–H⋯S hydrogen bond through enzyme assays on wild-type and mutant MetAP proteins from Mycobacterium tuberculosis that show a drastic difference in catalytic efficiency. The X-ray crystallographic structure of the methionine bound protein revealed a conserved water bridge and short contacts involving the Met side-chain, a feature also observed in MetAPs from other organisms. Thermal shift assays showed a remarkable 3.3 °C increase in melting temperature for methionine bound protein compared to its norleucine homolog, where C–H⋯S interaction is absent. The presence of C–H⋯S hydrogen bonding was also corroborated by nuclear magnetic resonance spectroscopy through a change in chemical shift. Computational chemistry studies revealed the unique role of the electrostatic environment in facilitating the C–H⋯S interaction. The significance of this atypical hydrogen bond is underscored by the fact that the function of MetAP is essential for any living cell.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...