The reactivation of tabun-inhibited mutant AChE with Ortho-7: steered molecular dynamics and quantum chemical studies†
Abstract
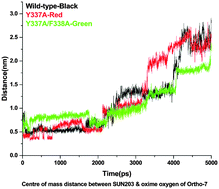
A highly toxic nerve agent, tabun, can inhibit acetylcholinesterase (AChE) at cholinergic sites, which leads to serious cardiovascular complications, respiratory compromise and death. We have examined the structural features of the tabun-conjugated AChE complex with an oxime reactivator, Ortho-7, to provide a strategy for designing new and efficient reactivators. Mutation of mAChE within the choline binding site by Y337A and F338A and its interaction with Ortho-7 has been investigated using steered molecular dynamics (SMD) and quantum chemical methods. The overall study shows that after mutagenesis (Y337A), the reactivator can approach more freely towards the phosphorylated active site of serine without any significant steric hindrance in the presence of tabun compared to the wild type and double mutant. Furthermore, the poor binding of Ortho-7 with the peripheral residues of mAChE in the case of the single mutant compared to that of the wild-type and double mutant (Y337A/F338A) can contribute to better efficacy in the former case. Ortho-7 has formed a greater number of hydrogen bonds with the active site surrounding residues His447 and Phe295 in the case of the single mutant (Y337A), and that stabilizes the drug molecule for an effective reactivation process. The DFT M05-2X/6-31+G(d) level of theory shows that the binding energy of Ortho-7 with the single mutant (Y337A) is energetically more preferred (−19.8 kcal mol−1) than the wild-type (−8.1 kcal mol−1) and double mutant (Y337A/F338A) (−16.0 kcal mol−1). The study reveals that both the orientation of the oxime reactivator for nucleophilic attack and the stabilization of the reactivator at the active site would be crucial for the design of an efficient reactivator.

 Please wait while we load your content...
Please wait while we load your content...