Study on the inter- and intra-peptide salt-bridge mechanism of Aβ23–28 oligomer interaction with small molecules: QM/MM method†
Abstract
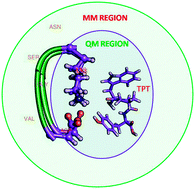
Amyloid β (Aβ) peptides have long been known to be a potential candidate for the onset of Alzheimer's disease (AD). The biophysical properties of Aβ42 peptide aggregates are of significant importance for the amyloid cascade mechanism of AD. It is necessary to design an inhibitor using small molecules to reduce the aggregation process in Aβ42 peptides. Attention has been given to use the natural products as anti-aggregation compounds, directly targeting Aβ peptides. Polyphenols have been extensively studied as a class of amyloid inhibitors. 9,10-Anthraquinone (AQ) is present in abundance in medicinal plants (rhubarb), the Trp–Pro–Tyr (TPT) peptide has been found in the venom of the black mamba snake, and the morin molecule is naturally present in wine and green tea; several other polyphenol derivatives are under clinical trials to develop anti-neurodegenerative drugs. In vitro and in vivo results strongly suggest that AQ and morin molecules are potential inhibitors of Aβ aggregation; however, the detailed understanding of the inhibition mechanism remains largely unknown. The formation of Aβ fibrils and oligomers requires a conformational change from α-helix to β-sheet, which occurs due to the formation of a salt-bridge between Asp23 and Lys28 residues. The present study focused on investigating the salt-bridge mechanism in the monomer, dimer and oligomer of the Aβ23–28 peptide during the interaction with TPT, morin and AQ molecules. Interaction energy and natural bond orbital analyses have been carried out using the ONIOM(M05-2X/6-31++G(d,p):UFF) method. The QM/MM studies have been performed to study the mechanism of salt-bridge formation during the inhibition process of amyloid β protein aggregation. The TPT molecule, which binds with the Asp23 and Lys28 residues of Aβ, prevents the salt-bridge formation between Asp23 and Lys28 residues and consequently the probability of the formation of Aβ fibrils is reduced.

 Please wait while we load your content...
Please wait while we load your content...