Effects of the A117V mutation on the folding and aggregation of palindromic sequences (PrP113–120) in prion: insights from replica exchange molecular dynamics simulations†
Abstract
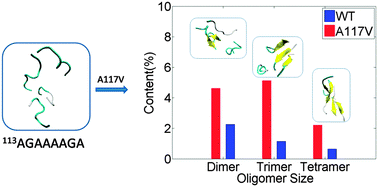
The palindromic region AGAAAAGA (PrP113–120) in prion is highly amyloidogenic and very critical in the structural conversion of cellular prion protein to its pathogenetic form. In this region, there is an important point mutation A117V, which is closely related to the occurrence of Gerstmann–Straussler–Scheinker Syndrome. However, the detailed knowledge about the effects of the A117V mutation on the folding and aggregation of the palindromic sequences is still lacking. To investigate the impacts of A117V mutation on the earliest steps along the PrP113–120 aggregation pathway, replica exchange molecular dynamics simulations of the monomer, 2- and 4-peptide systems of PrP113–120 and its A117V mutant were carried out. The simulations of monomers indicate that both WT and the A117V mutated PrP113–120 are mostly random coils with helical structures transiently populated. Differently, the A117V mutation enhances the intrinsic disorder of PrP113–120. The simulations of 2- and 4-peptide systems of the two species show that the A117V mutation increases the sheet contents and the populations of oligomers, which may be attributed to the enhancement of inter-peptide backbone hydrogen bonding interactions and side chain hydrophobic interactions. Overall, the study provides structural insights into the impacts of the A117V mutation on the folding and assembly of the palindromic sequences, which might be helpful to elucidate the mechanism underlying prion disease and the origin of the Gerstmann–Straussler–Scheinker Syndrome.

 Please wait while we load your content...
Please wait while we load your content...