Binding structures and energies of the human neonatal Fc receptor with human Fc and its mutants by molecular modeling and dynamics simulations†
Abstract
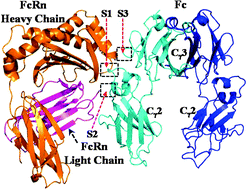
Homology modeling and molecular dynamics simulations have been carried out to model the detailed structures of the human neonatal Fc receptor (FcRn) binding with the wild-type Fc of human immunoglobulin G1 (IgG1) and its various mutants. Based on the modeled human FcRn–Fc binding structures, it has been proposed that the protein–protein binding interface is composed of three subsites. The first subsite is a hydrophobic core where residue I39 of human Fc can be accommodated very well, and the other two subsites are all composed of critical salt bridges between human FcRn and human Fc. All of the modeled structures and the calculated binding energies are qualitatively consistent with the available experimental data, suggesting that the modeled human FcRn–Fc binding structures are reasonable. The modeled human FcRn–Fc binding structure may be valuable for future rational design of novel mutants of human Fc and Fc-fused therapeutic proteins with a potentially higher binding affinity for human FcRn and, thus, a longer in vivo half-life in humans.

 Please wait while we load your content...
Please wait while we load your content...