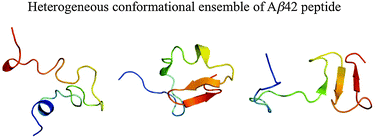
Alzheimer's disease is a neurodegenerative disorder characterized by progressive deposition of amyloid-beta (Aβ) peptides in brain parenchyma and cerebral blood vessels. Several pathogenic familial mutations of Aβ peptides have been identified that exhibit enhanced neurotoxicity and aggregative ability. However, knowledge of the structural characteristics of those Aβ mutants is still limited. Here, we report multiple all-atom molecular dynamics simulations of the wild-type 42-residue Aβ peptide (Aβ42) and its Flemish (A21G), Arctic (E22G), Dutch (E22Q), Italian (E22K), and Iowa (D23N) familial mutants in explicit water. After validating our simulations by comparison with available experimental data, we examined common/different features in the secondary and tertiary structures of the wild-type and five familial mutants of Aβ42. We found that Aβ42 peptides display quite heterogeneous secondary and tertiary structure ensembles. Such structural heterogeneity in the monomeric state would facilitate interconversions between various secondary structures during the formation of a β-sheet-rich amyloid fibril, and may also serve as a structural basis of the amyloid polymorphism.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...