Role of block copolymer-micelle nanocomposites in dye–bovine serum albumin binding: a combined experimental and molecular docking study†
Abstract
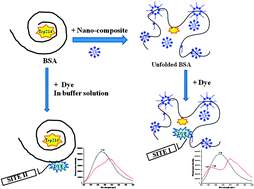
The role of a nanocomposite (NC), composed of intercalation of the diblock copolymer polyethylene-b-polyethylene glycol (PE-b-PEG) with the anionic surfactant sodium dodecyl sulphate (SDS), on the binding characteristics of bovine serum albumin (BSA) with a dye (1,8-naphthalimide, NAPMD) compared to the interaction between the same players in aqueous solution has been examined comprehensively in this paper. Static quenching due to complex formation in both NC medium and in buffer solution has been inferred on the basis of considerable changes in the absorption spectra of BSA on addition of NAPMD, of which the interaction is found to be stronger in NC medium. Temperature dependent fluorescence data also confirm an effective static quenching and stronger binding of NAPMD with BSA in NC medium. Peptide chain unfolding and denaturing of BSA in NC medium have been confirmed from steady state and time-resolved emission and circular dichroism data. This exposes both the tyrosine and tryptophan moieties as a unique case. Increased energy transfer between NAPMD and the tryptophan residue in the unfolded form of BSA helps in the appearance of tyrosine fluorescence in NC medium by quenching the tryptophan band. Ionization of the hydroxyl group in the aromatic ring of the tyrosine residue by the PEG group present in the NC medium produces a downshift of the tyrosine fluorescence band. The use of site selective markers confirms that NAPMD is near tryptophan in Sudlow’s site I in NC medium and in buffer solution it is away from tryptophan in Sudlow’s site II. The theoretical docking studies also vindicate the results of binding of NAPMD with BSA in site I or site II in NC and buffer media, as observed from different emission experiments including the site selective markers study.

 Please wait while we load your content...
Please wait while we load your content...