Supramolecular assemblies of a 1,8-naphthalimide conjugate and its aggregation-induced emission property†
Abstract
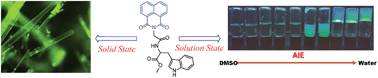
The aggregation-induced emission (AIE) property has attracted considerable research interest since its discovery. Several small molecule-based AIE fluorogens (AIEgens) have shown unique properties. In this work, we are reporting for the first time a 1,8-naphthalimide (NMI) conjugate of a dipeptide, which is found to exhibit J-type aggregation in the solid and solution state, and also in the gel state. Unsubstituted naphthalimide units usually exhibit aggregation-caused quenching due to face-to-face stacking compared to ring-substituted (V-shaped) derivatives. Here, the amide hydrogen bonding interaction present in the molecule guides the packing of unsubstituted naphthalimide units in the crystal structure and it is consistent with J-type aggregation. The self-assembly and gelation properties of the NMI derivative were studied in several pure and water-miscible mixed solvents. In the mixed solvents, gelation is caused by the anti-solvent effect. Aggregation-induced emission properties were observed upon addition of water in the water-miscible solvent solutions of the gelator. Finally, the AIE property of the NMI-peptide conjugate was used extensively for live-cell imaging.


 Please wait while we load your content...
Please wait while we load your content...