Nitrogen and sulfur co-doped fluorescent carbon dots for the trapping of Hg(ii) ions from water†
Abstract
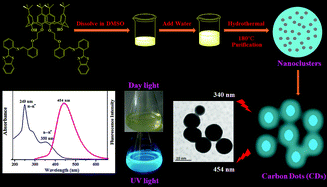
An uncomplicated, reliable, and ultrasensitive fluorescent sensor motif based on carbon dots (CDs) co-doped with nitrogen and sulfur atoms has been fabricated using calix[4]arene and benzothiazole moieties for the recognition of toxic mercuric ions (Hg2+) in aqueous medium and the application has been established in Tris–HCl buffer solution, real water samples, and the trapping of Hg2+ ions from aqueous medium. It was observed that the fluorescence intensity of the as-synthesized CDs could be entirely quenched in the presence of Hg2+ ions due to the electron/energy transfer process between the surface functional groups of the CDs and soft closed-shell Hg2+ ions. The proposed nanosensor could proficiently detect Hg2+ ions in aqueous medium with a detection limit of the order of 7.89 nM with high selectivity, sensitivity, and with a relatively low background interference even in a complex medium. The CDs were then further adsorbed on the surface of silica and were investigated for their ability to trap/capture Hg2+ ions using chromogenic and atomic absorption spectroscopy (AAS) method.
- This article is part of the themed collection: Fluorescent and Luminescent Materials


 Please wait while we load your content...
Please wait while we load your content...