Synthesis of stable gold nanoparticles using linear polyethyleneimines and catalysis of both anionic and cationic azo dye degradation†
Abstract
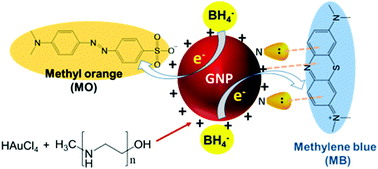
Reduction of auric acid with polyethyleneimine (PEI) provides a simple, low-cost alternative for the production of cationic gold nanoparticles (GNPs). However, linear PEI (lPEI) failed to produce small, colloidally stable GNPs, so far. Since lPEI is a polyelectrolyte, pH should be an important factor both in reduction and stabilization of GNPs and may be optimized to produce small and stable lPEI/GNPs. Cationic GNPs were produced by the direct reduction of auric acid in water with lPEI utilizing two different methods to dissolve the polymer: by protonation or at high temperature. The influence of pH on the particle formation and properties was studied over a wide pH range (3.5 to 10). The impacts of the PEI/Au mass ratio, polymer molecular weight (2.5 and 25 kDa) and post-synthetic pH on the particle properties were also studied. Best is to dissolve lPEI by protonation and to clean the GNPs via controlled centrifugal precipitation. The MW did not influence the hydrodynamic size, stability or particle shape, but low MW lPEI provided faceted particles. This simple one pot synthesis of small, stable cationic GNPs in water is a valuable, simple alternative for producing new cationic GNPs with even low molecular weight lPEI. Additionally, these GNPs were evaluated as catalysts in the degradation of methyl orange (MO) (anionic–zwitterionic) and methylene blue (MB) (cationic) azo dyes at different pH values. The fastest degradation of MO and MB was recorded at pH 7.5 and 3.5, respectively. Overall, this is a rare case where a single catalyst quickly and effectively catalyzes the degradation of both cationic and anionic dyes.


 Please wait while we load your content...
Please wait while we load your content...