High-efficiency and stable photocatalytic hydrogen evolution of rhenium sulfide co-catalyst on Zn0.3Cd0.7S†
Abstract
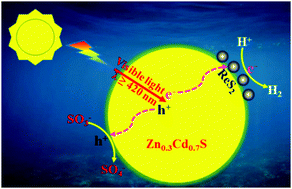
Photocatalytic hydrogen evolution is an attractive technology to solve the growing energy crisis. The development of advanced photocatalysts is an attractive but challenging issue. For the first time, we use inorganic ReS2 as a co-catalyst for Zn0.3Cd0.7S using a solvent thermal method, which enables highly efficient and recyclable H2 evolution. The Zn0.3Cd0.7S/ReS2 composite photocatalysts contain 4 wt% ReS2 co-catalysts, and Na2S–Na2SO3 is used as the sacrificial reagent. Compared with bare Zn0.3Cd0.7S, Zn0.3Cd0.7S/ReS2 corresponds to 101 fold enhancement with the highest photocatalytic H2 evolution rates of 92.45 mmol h−1 g−1. Furthermore, it still maintains high hydrogen production after a 30 hours cycle experiment. In this paper, the mechanism of photocatalytic enhancement is expounded by various experimental methods. In situ Fourier Transform Infrared (FT-IR) spectra and online mass spectrometry were used to discover the sources of hydrogen. The experimental results show that ReS2 is a promising joint catalyst for obtaining high-gloss catalytic hydrogen from water under visible light.


 Please wait while we load your content...
Please wait while we load your content...