Monolithic hydrogel nanowells-in-microwells enabling simultaneous single cell secretion and phenotype analysis†
Abstract
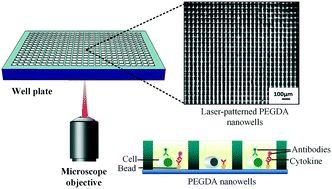
Cytokine secretion is a form of cellular communication that regulates a wide range of biological processes. A common approach for measuring cytokine secretion from single cells is to confine individual cells in arrays of nanoliter wells (nanowells) fabricated using polydimethylsiloxane. However, this approach cannot be easily integrated in standard microwell plates in order to take advantage of high-throughput infrastructure for automated and multiplexed analysis. Here, we used laser micropatterning to fabricate monolithic hydrogel nanowells inside wells in a microwell plate (microwells) using polyethylene glycol diacrylate (PEGDA). This approach produces high-aspect ratio nanowells that retain cells and beads during reagent exchange, enabling simultaneous profiling of single cell secretion and phenotyping via immunostaining. To limit contamination between nanowells, we used methylcellulose as a media additive to reduce diffusion distance. Patterning nanowells monolithically in microwells also dramatically increases density, providing ∼1200 nanowells per microwell in a microwell plate. Using this approach, we profiled IL-8 secretion from single MDA-MB-231 cells, which showed significant heterogeneity. We further profiled the polarization of THP-1 cells into M1 and M2 macrophages, along with their associated IL-1β and CCL-22 secretion profiles. These results demonstrate the potential to use this approach for high-throughput secretion and phenotype analysis on single cells.


 Please wait while we load your content...
Please wait while we load your content...