UniChip enables long-term recirculating unidirectional perfusion with gravity-driven flow for microphysiological systems†
Abstract
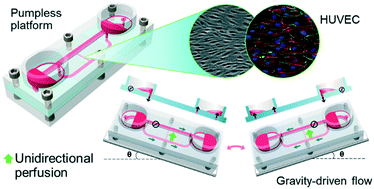
Microphysiological systems, also known as body-on-a-chips, are promising “human surrogates” that may be used to evaluate potential human response to drugs in preclinical drug development. Various microfluidics-based platforms have been proposed to interconnect different organ models and provide perfusion in mimicking the blood circulation. We have previously developed a pumpless platform that combines gravity-driven fluid flow and a rocking motion to create reciprocating flow between reservoirs for recirculation. Such platform allows design of self-contained and highly integrated systems that are relatively easy and cost-effective to construct and maintain. To integrate vasculature and other shear stress-sensitive tissues (e.g. lung and kidney) into pumpless body-on-a-chips, we propose “UniChip” fluid network design, which transforms reciprocating flow input into unidirectional perfusion in the channel(s) of interest by utilizing supporting channels and passive valves. The design enables unidirectional organ perfusion with recirculation on the pumpless platform and provides an effective backflow-proof mechanism. To demonstrate principles of UniChip design, we created a demonstration chip with a single straight channel as a simple example of the organ perfusion network. A BiChip providing bidirectional perfusion was used for comparison. Computational and experimental fluid dynamic characterization of the UniChip confirmed continuous unidirectional flow in the perfusion channel and the backflow-proof mechanism. Vascular endothelial cells cultured on UniChips for 5 d showed changes matching cell responses to unidirectional laminar flows. Those include cell elongation and alignment to the flow direction, continuous network of VE-cadherin at cell borders, realignment of F-actin and suppressed cell proliferation. Cells on BiChips manifested distinct responses that are close to responses to oscillatory flows, where cells remain a polygonal shape with intermittent VE-cadherin networks and few F-actin realignment. These results demonstrate that microfluidic devices of UniChip design provide recirculating unidirectional perfusion suitable for long-term culture of shear stress-sensitive tissues. This is the first time a gravity-drive flow system has achieved continuous unidirectional perfusion with recirculation. The inherent backflow-proof mechanism allows hassle-free long-term operation of body-on-a-chips. Overall, our UniChip design provides a reliable and cost-effective solution for the integration of vasculature and other shear stress-sensitive tissues into pumpless recirculating body-on-a-chips, which can expedite the development and widespread application of moderately high-throughput, high-content microphysiological systems.
- This article is part of the themed collections: Lab on a Chip Recent HOT Articles and Organ-, body- and disease-on-a-chip systems


 Please wait while we load your content...
Please wait while we load your content...
