Flash crystallization kinetics of methane (sI) hydrate in a thermoelectrically-cooled microreactor†
Abstract
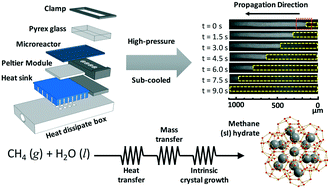
The crystallization kinetics of methane (sI) hydrate were investigated in a thermoelectrically-cooled microreactor with in situ Raman spectroscopy. Step-wise and precise control of the temperature allowed acquisition of reproducible data within minutes, while the nucleation of methane hydrates can take up to 24 h in traditional batch reactors. The propagation rates of methane hydrate (from 3.1–196.3 μm s−1) at the gas–liquid interface were measured for different Reynolds' numbers (0.7–68.9), pressures (30.0–80.9 bar), and sub-cooling temperatures (1.0–4.0 K). The precise measurement of the propagation rates and their subsequent analyses revealed a transition from mixed heat-transfer–crystallization-rate-limited to mixed heat-transfer–mass-transfer–crystallization-rate-limited kinetics. A theoretical model, based on heat transfer, mass transfer, and intrinsic crystallization kinetics, was derived for the first time to understand the non-linear relationship between the propagation rate and sub-cooling temperature. The molecular diffusivity of methane within a stagnant film (ahead of the propagation front) was discovered to follow Stokes–Einstein, while calculated Hatta (0.50–0.68), Lewis (128–207), and beta (0.79–116) numbers also confirmed that the diffusive flux influences crystal growth. Understanding methane hydrate crystal growth is important to the atmospheric, oceanic, and planetary sciences and to energy production, storage, and transportation. Our discoveries could someday advance the science of other multiphase, high-pressure, and sub-cooled crystallizations.


 Please wait while we load your content...
Please wait while we load your content...