Basic principles of electrolyte chemistry for microfluidic electrokinetics. Part I:† Acid–base equilibria and pH buffers‡§
Abstract
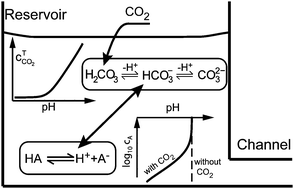
We review fundamental and applied acid–base equilibrium chemistry useful to microfluidic electrokinetics. We present elements of acid–base equilibrium reactions and derive rules for pH calculation for simple buffers. We also present a general formulation to calculate pH of more complex, arbitrary mixtures of electrolytes, and discuss the effects of ionic strength and temperature on pH calculation. More practically, we offer advice on buffer preparation and on buffer reporting. We also discuss “real world” buffers and likely contamination sources. In particular, we discuss the effects of atmospheric carbon dioxide on buffer systems, namely, the increase in ionic strength and acidification of typical electrokinetic device buffers. In Part II of this two-paper series, we discuss the coupling of acid–base equilibria with electrolyte dynamics and electrochemistry in typical microfluidic electrokinetic systems.
- This article is part of the themed collection: Fundamental Principles and Techniques in Microfluidics

 Please wait while we load your content...
Please wait while we load your content...