Millisecond denaturation dynamics of fluorescent proteins revealed by femtoliter container on micro-thermodevice
Abstract
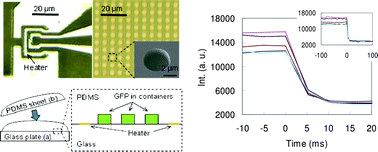
Real-time observation of biomolecular behavior focusing on high speed temperature response is an essential endeavor for further biological study at the molecular level. This is because most of the important biological functions at the molecular level happen at the sub-second time scale. We used our own on-chip microheaters and microcontainers to observe the denaturation dynamics of fluorescent

 Please wait while we load your content...
Please wait while we load your content...