Micromachined bulk PZT tissue contrast sensor for fine needle aspiration biopsy
Abstract
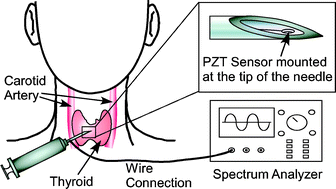
This paper describes a micromachined piezoelectric sensor, integrated into a cavity at the tip of a biopsy needle, and preliminary experiments to determine if such a device can be used for real-time tissue differentiation, which is needed for needle positioning guidance during fine needle aspiration (FNA) biopsy. The sensor is fabricated from bulk lead zirconate titanate (PZT), using a customized process in which micro electro-discharge machining is used to form a steel tool that is subsequently used for batch-mode ultrasonic micromachining of bulk PZT ceramic. The resulting sensor is 50 µm thick and 200 µm in diameter. It is placed in the biopsy needle cavity, against a steel diaphragm which is 300 µm diameter and has an average thickness of 23 µm. Devices were tested in materials that mimic the ultrasound characteristics of human tissue, used in the training of physicians, and with porcine fat and muscle tissue. In both schemes, the magnitude and frequency of an electrical impedance resonance peak showed tissue-specific characteristics as the needle was inserted. For example, in the porcine tissue, the impedance peak frequency changed ≈13 MHz from the initial 163 MHz, and the magnitude changed ≈1600 Ω from the initial 2100 Ω, as the needle moved from fat to muscle. Samples including oils and saline solution were tested for calibration, and an empirical tissue contrast model shows an approximately proportional relationship between measured frequency shift and sample acoustic impedance. These results suggest that the device can complement existing methods for guidance during biopsies.

 Please wait while we load your content...
Please wait while we load your content...