Alternately deposited heterostructures of α-sexithiophene–para-hexaphenyl on muscovite mica(001) surfaces: crystallographic structure and morphology
Abstract
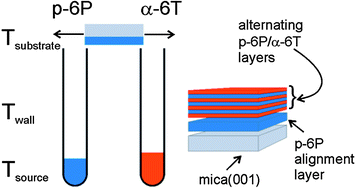
Multi-component systems of para-hexaphenyl (p-6P) and α-sexithiophene (α-6T) molecules show great promise for tuning the fluorescence colour of optically active films. As the opto-electronic properties of rod-like molecules in thin films strongly rely on their anisotropic orientation, a technique for preparation of well-defined, anisotropic multicomponent systems is required. We demonstrate that a p-6P film of less than two nanometer thickness grown on muscovite mica(001) substrates acts as an efficient alignment layer for epitaxial growth of α-6T crystallites. On top of such a p-6P alignment layer, multilayer heterostructures of alternately deposited p-6P and α-6T molecules were grown. Combined

 Please wait while we load your content...
Please wait while we load your content...