One dimensional Ag/Au/AgCl nanocomposites stemmed from Ag nanowires for electrocatalysis of oxygen reduction†
Abstract
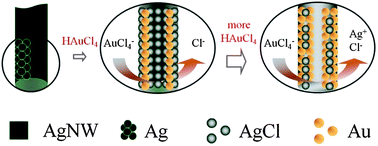
This paper reports the synthesis and characterization of one dimensional nanocomposites composed of Ag, Au, and AgCl (denoted as Ag/Au/AgCl). The Ag/Au/AgCl composites with different compositional ratios are prepared by the galvanic replacement reaction between a Ag nanowire template and Au precursor at various concentrations (1, 10, 100 mM HAuCl4). As the Au precursor concentration increases, the structure of the resulting Ag/Au/AgCl changes from a core–shell solid wire to a porous hollow wire with a decrease in the Ag and AgCl contents and increase in the Au content. The catalytic activity of Ag/Au/AgCl for the oxygen reduction reaction (ORR) is investigated in 0.1 M NaOH solution. The nanocomposite with a Ag : Au : AgCl compositional ratio of 4 : 86 : 10 shows reasonable ORR activity (high ORR current, positive onset potential) along with sufficient stability and

 Please wait while we load your content...
Please wait while we load your content...