First-principles study of the magnesiation of olivines: redox reaction mechanism, electrochemical and thermodynamic properties†
Abstract
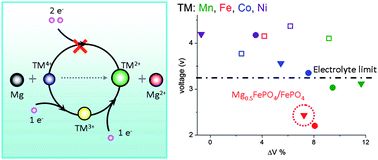
Development of Mg batteries relies heavily on the thermodynamics and kinetics of the magnesiation of cathode materials that favor the high mobility of Mg ions in the host lattice and energy densities at least comparable to Li ion batteries. In this paper, we performed Density Functional Theory studies to understand the thermodynamics of the magnesiation of olivine compounds. The redox reaction mechanism was revealed in the magnesiation process of MnSiO4. Mn4+ in the host structure was first reduced to Mn3+ resulting in the formation of Mg0.5MnSiO4. Further

 Please wait while we load your content...
Please wait while we load your content...