Sulfur and selenium substituted spiro-biphenalenyl-boron neutral radicals†
Abstract
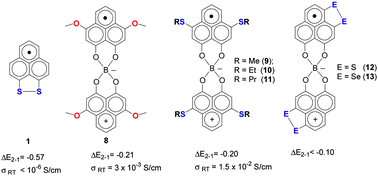
We report a synthetic scheme for the preparation of alkylthio, dithio-bridged and diseleno-bridged 9-hydroxyphenalenones and associated spiro-biphenalenyl boron neutral radicals. We show that the strategy of sulfur substitution at the active positions of the phenalenyl units reduces the electrochemical disproportionation potential (ΔE2−1 = E2½ − E1½, where E1½ and E2½ are the first and second reduction potentials of corresponding cations) of the alkylthio-radicals [3,7-SR-PLY(O,O)]2B, (R = Me, 9), (R = Et, 10) and (R = Pr, 11), but brings about a significant reduction of the ΔE2−1 value in the case of disulfide and diselenide substitution, [3,4-S,S-PLY(O,O)]2B (12) and [3,4-Se,Se-PLY(O,O)]2B (13). The crystal structures of 10 and 11 show that the radicals exist as one dimensional (1-D) π-chains of superimposed phenalenyl units, and the molecular units pack more efficiently than the oxygen-substituted analog [3,7-OMe-PLY(O,O)]2B (8). Magnetic susceptibility measurements indicate that in the solid state the radicals remain paramagnetic while there is spin–spin interaction between the molecules along the π-chains. Band structure calculations are in accordance with the magnetic measurement data and indicate the presence of interactions between the molecules. The room temperature electrical conductivities of both compounds are found to be σRT = 1.5 × 10−2 S cm−1.

 Please wait while we load your content...
Please wait while we load your content...