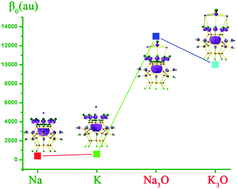
It is well known that electrides are a type of multielectron many-cage solid salt with excess electron anions inside the cages. The main concern regarding these structures is how to construct the organic single-caged electride molecules with an electron inside its cage. Using the perfluorinated fullerene cage C20F20 as the electron hole, the alkali metal atoms (M = Na, K) and superalkali atoms (M3O, M = Na, K) with a smaller vertical detachment energy (VDE) value as the source of the electrons, we can construct new nonlinear optical (NLO) organic single-caged electride salt molecules M+(e@C20F20)− and (M3O)+(e@C20F20)− due to the long-range charge transfer from the (super)alkali to inside the cage, forming an electron-hole pair within the molecule. To measure the nonlinear optical response, static first hyperpolarizabilities (β0) and the superalkali effect on β0 are exhibited for these new molecules. The β0 values are 400 and 600 au for M+(e@C20F20)− which are considerably smaller than 13 000 and 10 000 au for (M3O)+(e@C20F20)−. It is revealed that the superalkali effect on the β0 value is dramatic and the β0 value increases by about 20–30 times. New single-caged superalkali electride salt molecules (M3O)+(e@C20F20)− possess not only a large nonlinear optical property but also higher stability. They hold potential as high-performance nonlinear optical materials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...