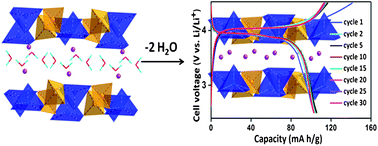
The layer structured αI-LiVOPO4 was obtained via a two step chemical synthesis. In the first step, a hydrated phase, LiVOPO4·2H2O, was obtained by a simple hydrothermal route at 120 °C. Single crystal X-ray diffraction analysis revealed the structure of LiVOPO4·2H2O to be orthorhombic with lattice parameters: a = 8.9454(7) Å, b = 9.0406(7) Å and c = 12.7373(10) Å. Dehydration of the parent compound led to its structural transformation to tetragonal αI-LiVOPO4, which was only identified previously during the lithium insertion in VOPO4. We have investigated the solid-state dehydration of LiVOPO4·2H2O and proposed a possible mechanism for the crystal structure transformation. Electrochemical characterization of this rarely studied tetragonal phase revealed its good lithium cycling at high operating voltage. Galvanostatic charge–discharge cycling of αI-LiVOPO4 was studied in a voltage window of 2.5–4.5 V, which shows a stable reversible capacity of 103(±3) mA h g−1 at a current density of 16 mA g−1 (0.1 C). At higher current rates, although it exhibited good cyclability, the capacity was found to decrease with increasing current rates. The long term cycling stability of the above material was demonstrated at a current rate of 0.5 C up to 200 cycles.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...